
(1970), The Mathematical Theory of Non-Uniform Gases (3rd ed.), Cambridge University Press In the following table, the temperature is given in kelvins. Viscosity of water as a function of temperature Substance Substances of variable composition Substance s) of aqueous solutions at T = 20 ☌ for various solutes and mass percentages.The increase in viscosity for sucrose solutions is particularly dramatic, and explains in part the common experience of sugar water being "sticky". For instance, the table below shows that viscosity increases monotonically with concentration for sodium chloride and calcium chloride, but decreases for potassium iodide and cesium chloride (the latter up to 30% mass percentage, after which viscosity increases). The viscosity of an aqueous solution can either increase or decrease with concentration depending on the solute and the range of concentration. This is also the reason oils tend to be highly viscous, since they are usually composed of long-chain hydrocarbons. More dramatically, a long-chain hydrocarbon like squalene (C 30H 62) has a viscosity an order of magnitude larger than the shorter n-alkanes (roughly 31 mPa This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. One of the key predictions of the theory is the following relationship between viscosity μ For this reason, measured viscosities of the noble gases serve as important tests of the kinetic-molecular theory of transport processes in gases (see Chapman–Enskog theory). The simple structure of noble gas molecules makes them amenable to accurate theoretical treatment.

By contrast, pressure is omitted since gaseous viscosity depends only weakly on it. The temperatures corresponding to each data point are stated explicitly. Where data points are unavailable for 25 ☌ or 1 atmosphere, values are given at a nearby temperature/pressure. Here "standard conditions" refers to temperatures of 25 ☌ and pressures of 1 atmosphere. Viscosities at or near standard conditions

1 Viscosities at or near standard conditions.The main objective of this study is to demonstrate effects of water as well as temperature on viscosity of aqueous glycerol solutions, applying experimental data of accumulated amounts of aqueous glycerol solutions at various drain durations to the newly-developed viscosity equation for the fabricated tank-tube viscometer. Very viscous glycerol and its aqueous solutions were used to test the viscometer by comparing viscosity values from the viscometer with those from literatures. The kinetic energy of the emerging stream from the bottom end of the vertical tube of the tank-tube viscometer also is assumed to be negligible. Both the quasi steady state approach and the negligible friction loss due to a sudden contraction between the reservoir tank and the tube are valid. The viscosity equation was developed under the following assumptions. The tank-tube viscometer consists of a large-diameter reservoir and a long, small-diameter, vertical tube. This inexpensive viscometer continuously generates numerous reproducible viscosity data of highly viscous neat glycerol and its aqueous solutions under given experimental conditions such as a desired temperature and a desired concentration of water in aqueous glycerol solutions.įabricating the tank-tube viscometer is inexpensive, since this viscometer does not need sophisticated accessories such as a high-pressure liquid pump, a sensitive pressure sensor, and an accurate flow meter.
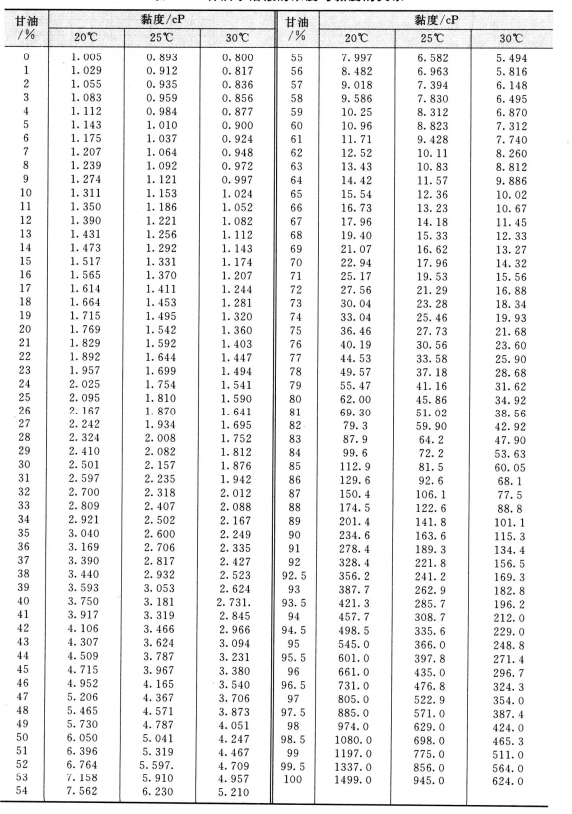
Measuring viscosity of highly viscous liquids with the tank-tube viscometer is easier than other types of viscometers.
#Viscosity of glycerol series
In this paper we demonstrate several series of experiments for the measurement of viscosity of neat glycerol and its aqueous solutions using a tank-tube viscometer.


 0 kommentar(er)
0 kommentar(er)
